If your business operates in a regulated industry, translation is not just “converting text.” It becomes part of your compliance system. Regulatory compliance for translated documents must be linguistically accurate, legally valid, and ready for audits. That means it follows well-structured workflows, protects sensitive data, and aligns with relevant standards and regulations, including ISO 17100, GDPR, HIPAA, FDA/EMA expectations, and financial disclosure requirements.
Here, we explain what compliance means in translation, where it applies, and how organizations can reduce risk through the right workflow and vendor.
What is Regulatory Compliance
Regulatory compliance means following the laws, rules, and official standards that apply to your business or industry. These requirements can come from various sources, such as government regulators, industry bodies, and internal policies.
In translation work, regulatory compliance means your translated documents are accurate, legally valid (when required), secure, and ready for audits. This is especially important when the content supports decisions, safety, patient care, financial reporting, or legal obligations.
What Does Regulatory Compliance Mean in Translation
We often hear the question: “What is regulatory compliance in the context of translated documents?” In simple terms, it means your translated content complies with the rules that apply to your industry, region, and use case, without creating legal, privacy, or audit issues.
In translation, compliance usually means the translation stays accurate and legally valid, so the meaning remains correct across obligations, warnings, and technical terms. It also requires robust confidentiality and privacy protections.
As a result, sensitive data is handled securely and lawfully. Just as important is traceability; you should be able to show who translated, reviewed, and approved. Also, deliver the document, along with audit readiness documentation, showing how your workflow produces records such as version history, QA logs, and approvals that regulators or auditors may request.
Finally, compliance depends on process consistency, ensuring that terminology, formatting, and revisions remain consistent across versions and languages. For these reasons, translation for regulated industries requires more than basic proofreading. It needs documented controls.
5 Key Regulatory Drivers for Multilingual Compliance
When your company runs across borders, compliance isn’t only about the original document. The translated version often needs to comply with the same legal, privacy, and formatting requirements. Because regulators, auditors, and local teams rely on what they can read.
Here are the 5 key points that drive the push of organizations to use regulatory translation and controlled workflows:
- Data privacy and confidentiality rules (GDPR, HIPAA, etc.)
If a document contains personal, patient, or financial data, the translation process must protect that data through secure handling, limited access, and audit-friendly controls. - Regulated submissions and approvals (FDA/EMA and similar bodies)
Clinical, pharma, and medical device documentation often requires strict terminology, traceable review steps, and consistent formatting to avoid delays or rejection. - Financial reporting and disclosure standards (SEC/FINRA and equivalents)
Investor-facing documents must be consistent across languages. A shift in wording in risk disclosures or forward-looking statements can create legal exposure. - Legal enforceability and cross-border validity
Contracts, court filings, and compliance notices may require certified or sworn translations, depending on jurisdiction and the document’s use. - Safety, labeling, and product compliance (CE/OSHA and local regulations)
Instructions, warnings, and safety manuals must be clear and consistent. A translation error in a safety instruction can trigger audits, claims, or recalls. - Audit readiness and traceability requirements
Many regulated organizations need proof of who translated what, which version was used, what changed, and who approved it, especially during audits or disputes.
Which Industries Require Strict Regulatory-Compliant Translations
Regulatory requirements differ by sector. But some industries consistently need strict controls, documentation, and security, such as legal (cross-border contracts, court filings, dispute materials), healthcare and pharma (patient records, consent forms, clinical trial documents), medical devices (IFU, labeling, safety warnings, technical files), finance (annual reports, disclosures, investor packs, audit materials), insurance (policy wording, claims documents, customer disclosures), manufacturing and engineering (safety manuals, compliance documents, technical procedures), and government/public sector (official notices, tenders, policy documents, citizen-facing forms).
For example, a global team may need translations for FDA submissions or EMA documentation, HIPAA-related medical records, financial reports tied to disclosure rules, or safety manuals aligned with OSHA/CE. Each sector has its own “must-have” compliance requirements.
Common Legal Translation Errors That Create Business Risk
Most legal and compliance errors caused by translation follow the same pattern: speed or cost is prioritized over legal accuracy. Legal content or documents always require specialist handling. General translation workflows are not designed to protect enforceability, jurisdictional meaning, or regulatory intent.
At Circle Translations, legal contract translation is treated as a controlled and risk-aware process. Every project is handled by legal translators with subject-matter expertise and supported by structured quality controls suited for high-risk documents.
Misinterpreting Legal Terms and Contract Intent
Legal terms do not work the same way across jurisdictions. Most problems arise when translators focus solely on literal meaning rather than on how a term functions within a specific legal system. A small wording error can change liability, weaken protections, or create obligations that were never intended.
These risks increase when contracts are translated by non-specialists or through automated tools without legal review.
We try to reduce this risk by assigning legal translators with proven experience in contracts, regulations, and jurisdiction-specific terminology.
Missing Jurisdiction and Legal-System Differences
Legal language is closely tied to local laws and enforcement practices. A translation may seem correct linguistically but still fail legally if it does not align with the target jurisdiction’s legal framework. This often leads to rejected filings or unenforceable clauses.
Our legal translators work with jurisdictional awareness, ensuring that translated contracts reflect how terms are interpreted and applied in the target legal system.
Structural and Consistency Errors
Missing clauses, inconsistent terminology across related agreements, or incorrect numbering can create serious compliance gaps. These issues usually come from fragmented workflows or limited review stages.
Circle Translations addresses this through multi-layer quality assurance. Each legal translation undergoes structured revision, terminology checks, and consistency validation to ensure that the full legal meaning of the source document is preserved without loss or distortion.
This controlled method helps global teams reduce legal exposure and maintain confidence in their translated contracts.
Key Requirements for Compliant Translations
Regulatory-compliant work is built on repeatable controls. Most organizations require:
- Qualified linguists with proven domain experience
- Documented QA and linguistic quality assurance (LQA)
- Terminology and style control
- Secure handling of files and sensitive data
- Version control and audit trails
What standards govern compliant translation?
For legal documents translations, some govt. Set some standards. Here’s a simple overview of major standards and why they matter:
- ISO 17100: process requirements for quality translation services (qualified translators, revision, documented workflows)
- GDPR: rules for handling personal data in the EU/EEA (privacy, access controls, lawful processing)
- HIPAA: protection of patient health information (PHI) in the U.S. healthcare context
- FDA/EMA: expectations for clinical, pharma, and medical documentation (clarity, consistency, submission-ready formatting)
- SEC/FINRA: requirements for financial reporting and disclosure communications in regulated financial environments
- CE/OSHA (and related safety frameworks): safety labeling and manuals that must be clear, consistent, and accurate
Your compliance team may also need to assign a certified regulatory compliance manager when translations are included in regulated submissions, audits, or high-risk customer documentation.
Role of Certified or Sworn Translations in Compliance
Honestly, not every regulated document needs a certified or sworn translation. But some do, especially when the translated document must be legally accepted by an authority or government body.
Certified or sworn translations are commonly needed for:
- Court filings and litigation documents
- Immigration and official government submissions
- Certain cross-border contracts and notarized documentation
- Financial audits and formal statements
- Medical or clinical documentation in specific jurisdictions
In these cases, the translation may include a certificate of accuracy, the translator’s credentials, and formal statements that meet local legal requirements.
Step-by-step Guide of Compliance Workflow
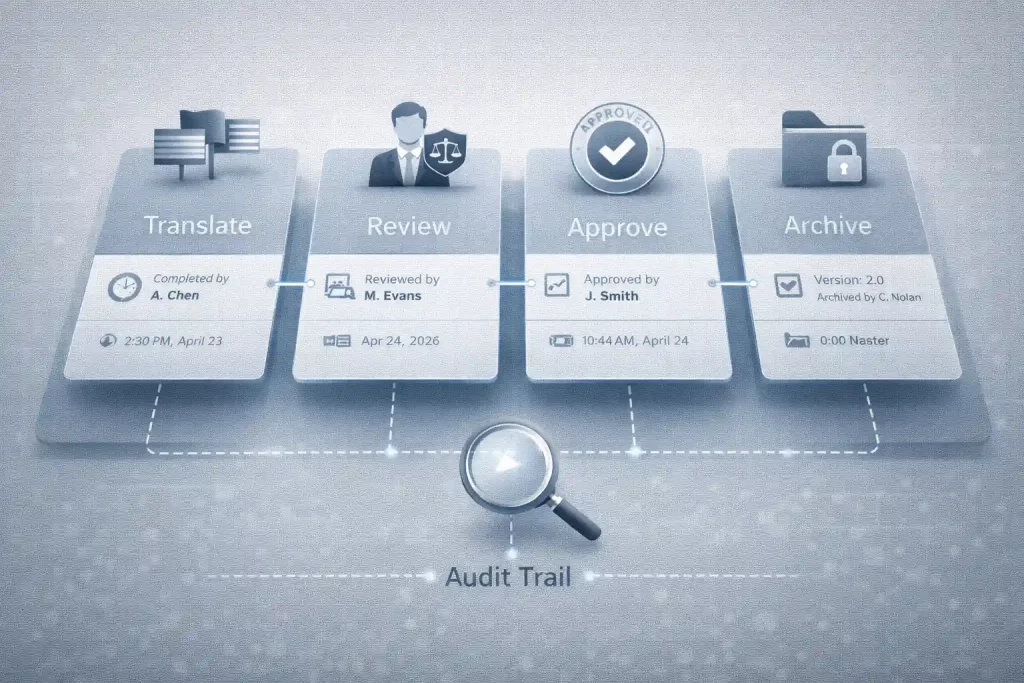
A compliant workflow is structured, repeatable, and documented. Below, we outline the workflow step by step.
Step 1: Document intake & compliance scoping
At intake, the goal is to define compliance needs before any translation begins. This includes:
- Document type and purpose (submission, internal, customer-facing, court, audit)
- Jurisdictions and regulations involved
- Certification or sworn translation requirements
- Privacy requirements and data sensitivity
- Formatting needs and deadline expectations
This step answers the practical question: how do I ensure compliance in enterprise translations without wasting time later?
Step 2: Assigning qualified subject-matter linguists
Regulated documents should be translated by linguists who are:
- Qualified and experienced in the specific industry
- Trained to follow compliance workflows
- Comfortable with regulated terminology and document structure
In higher-risk work, teams may add legal or technical reviewers depending on jurisdiction and the document’s role.
Step 3: Controlled terminology & style
Before production, terminology is aligned through:
- Approved glossaries and termbases
- Style guides and formatting rules
- Version control for templates and repeated clauses
- Clear guidance on non-translatable items (product names, legal references, standards)
This is one of the strongest controls in regulatory translation because it prevents “silent drift” across documents and regions.
Step 4: Translation + revision + LQA
Compliance work typically requires more than one pass. A common structure is:
- Translation by a qualified linguist
- Independent revision (second linguist)
- Linguistic QA (terminology, consistency, completeness)
- Format validation (tables, numbering, references, templates)
This layered review reduces the risk of errors that only surface later. During audits, disputes, or regulatory feedback.
Step 5: Certification, sworn validation, or legal formatting
If certification is required, the translation is prepared with the correct statements, stamps, and supporting documentation for the jurisdiction.
This step can also include formatting rules for regulated submissions—such as consistent page numbering, locked tables, template alignment, and controlled references.
Step 6: Secure delivery, audit logs, and archiving
Finally, delivery and retention must support audit readiness:
- Secure export and encrypted delivery
- Controlled access to final files
- Archived project records and QA logs
- Clear version history for future updates or resubmissions
How Non-compliance Creates Risk for Organizations
When translated documents fail to meet regulatory requirements, the outcome goes far beyond simple corrections. In regulated environments, non-compliance can disrupt operations, trigger legal action, and expose organizations to long-term risk.
At Circle Translations, we see these issues most often when translation is treated as a one-off task instead of a controlled compliance process.
Rejected filings and regulatory delays
Regulators assess submissions based on the translated content they receive. If the terminology, formatting, or certifications are incorrect, filings may be rejected or returned for revision. This leads to re-submission delays that slow approvals, product launches, or market entry.
Legal penalties and enforcement exposure
Non-compliant translations can trigger fines, enforcement actions, or formal investigations. Even when the source document is correct, errors in the translated version can be treated as regulatory violations.
Product recalls and safety risk
Incorrect translations in instructions, labels, or warnings can result in product recalls. In sectors like medical devices, manufacturing, and pharma, these errors directly affect user safety and regulatory trust.
Financial and disclosure risk
Inaccurate translations of financial reports or disclosure documents can lead to restatements, audit issues, or investor disputes. Regulators and stakeholders rely on translated financial information to be complete and precise.
Operational disruption in insurance and clinical workflows
Insurance claim disputes and clinical trial delays often stem from unclear or non-compliant translated documentation. Missing details or inconsistent terminology can place claims on hold or suspend trial activity.
Circle Translations helps organizations reduce these risks by treating compliance translation as a structured, auditable process, designed to meet regulatory expectations, protect operations, and support long-term governance.
Can AI or machine translation be used safely in compliance workflows?
Yes, but only in controlled conditions. In this era, it is very common and sometimesan easy way to use AI for document translation. But when it comes to legal or compliance work, it should be a bit more controlled and manualized than the other workflow.
AI or machine translation can help with drafts or low-risk internal materials. But regulated content still requires:
- Human review and independent revision
- Secure, enterprise-grade tools (not public/open tools for sensitive data)
- Clear policies for what content can and cannot be processed
- Auditability (who used what tool, on which version)
In regulated environments, AI can be a support layer, not the final authority.
How Circle Translations Supports Regulatory Compliance for Translation Documents
If your organization or team needs audit-ready regulatory translation, the goal is simple: reduce risk while maintaining reliable delivery.
Our legal translation model supports regulated teams with:
- ISO-aligned workflows and documented QA
- Qualified, subject-matter linguists
- Terminology management and version control
- Secure file handling with controlled access
- Audit-ready records (logs, approvals, delivery history)
- Certification or sworn translation support when required
Lastly, Regulatory translation is not just about language. It’s about accuracy, traceability, and security that can withstand audits and legal scrutiny. If your documents support regulated work (legal, medical, pharma, finance, insurance, manufacturing, or public sector), a structured compliance workflow with qualified linguists, terminology control, and secure handling is the safest way to reduce risk and avoid costly rework.
Let’s connect to get hassle-free legal document translation for your team.
Frequently Asked Questions
What does “regulatory-compliant translation” mean?
Regulatory-compliant translation means the translations that meet legal, industry, privacy, and process standards, such as ISO 17100, GDPR, HIPAA, FDA/EMA regulations, and financial reporting standards. They must be accurate, traceable, and audit-ready.
Do all regulatory documents require certified translations?
Not always. Court filings, immigration documents, financial audits, and some medical or pharma-related submissions may require certified or sworn translations. But other documents may only require an ISO-aligned workflow with revision, LQA, and traceability.
Can machine translation be used for regulated content?
Yes, but with conditions. You can use machine translations only in controlled workflows. Regulated sectors usually require human review and secure enterprise-grade MT. Sensitive data (legal, patient, financial) should not be processed through public tools.
What happens if translated documents do not meet compliance requirements?
Non-compliance can trigger rejected submissions, disputes, fines, product recalls, or privacy penalties. Regulators may request resubmission or initiate audits, which is time consuming and sometimes costly for an organization.
How do I know if a translation provider is compliance-ready?
Look for ISO 17100 processes, secure portals, certified/sworn translator options, audit logs, privacy controls, documented QA steps, and proven experience in your industry.
Which industries have the strictest compliance translation rules?
Healthcare/pharma, finance, legal, insurance, government, and regulated manufacturing typically have the strictest requirements due to privacy rules, safety risk, and formal submission standards.
What information does a vendor need to deliver compliant translations?
File type, jurisdictions, applicable regulations, required certifications, deadlines, privacy constraints, approved terminology, previous submissions (if relevant), and formatting rules.